Photobiomodulation for Cognitive Dysfunction (Brain Fog) in Post-COVID-19 Condition: A Randomized Sham-Controlled Pilot Trial
Post-COVID-19 “brain fog” remains one of the most disabling and stubborn symptoms of long COVID. Up to 88% of people with post-COVID-19 condition (PCC) report cognitive issues affecting attention, memory, and executive function, yet there are still no proven treatments targeting this aspect of the syndrome.
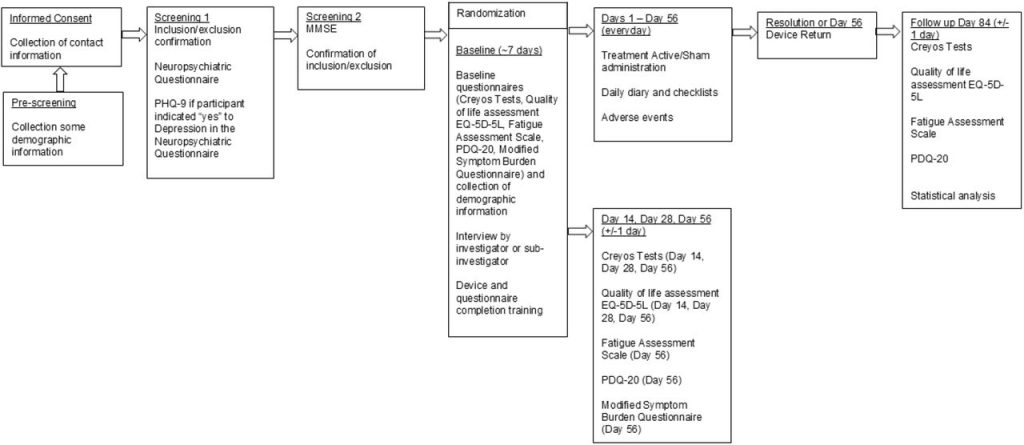
A new randomized, double-blind, sham-controlled clinical trial (n=43) has now explored whether home-based photobiomodulation (PBM) using the Vielight Neuro RX Gamma device can help improve cognition in adults with PCC-related brain fog.
Why Photobiomodulation for Long-COVID Brain Fog?
PCC-related cognitive dysfunction is thought to arise from a combination of:
-
Neuroinflammation and microglial activation
-
Blood–brain barrier disruption
-
Impaired default mode network (DMN) connectivity
-
Mitochondrial dysfunction and impaired cellular energy production
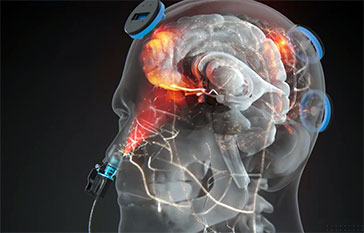
Photobiomodulation uses near-infrared light (typically 600–1100 nm) to modulate biological pathways, supporting mitochondrial function, ATP production, and anti-inflammatory signaling. In the brain, 40 Hz gamma-frequency PBM has been linked to beneficial modulation of neural oscillations and microglial activity, with potential downstream effects on cognition and neuroprotection.
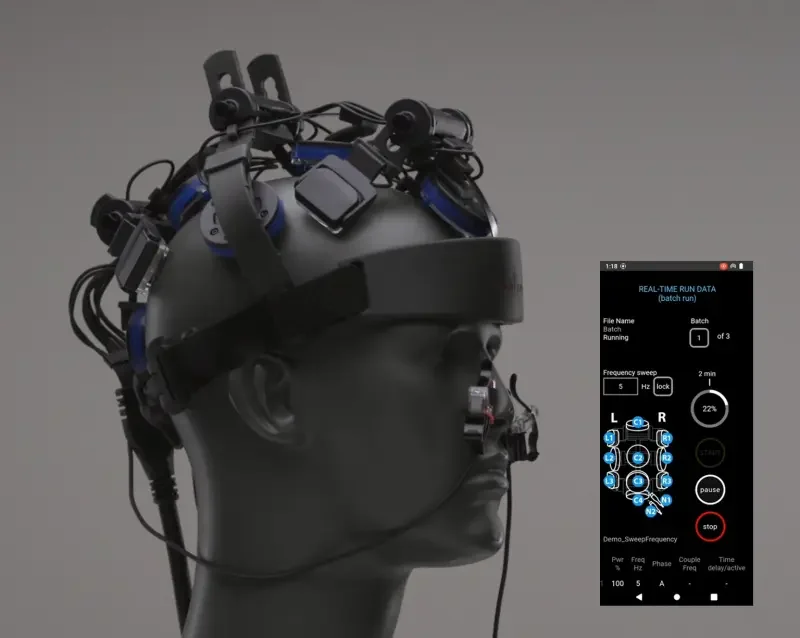
The Vielight Neuro RX Gamma delivers 810 nm near-infrared light pulsed at 40 Hz both transcranially and intranasally (an intranasal + transcranial, or “itPBM,” approach), targeting hubs of the default mode network and cerebellum. This trial tested whether that targeted, home-based itPBM could improve cognitive performance in people living with long COVID brain fog.
Study Design at a Glance
-
Design: Prospective, randomized, double-blind, sham-controlled pilot trial (CONSORT-compliant)
-
Registration: ClinicalTrials.gov NCT05857124
-
Setting: Fully remote, home-based study with online assessments and electronic data capture
-
Participants:
-
Adults 18–65 years
-
Met WHO criteria for PCC with cognitive symptoms ≥12 weeks post-COVID
-
Documented prior SARS-CoV-2 infection
-
Excluded: major neurological/psychiatric disease, significant brain injury, unstable illness, pregnancy, photosensitivity, seizure risk, or cognition-impairing substances
-
A total of 43 participants were randomized:
-
23 to active PBM (Vielight Neuro RX Gamma v2)
-
20 to sham
Most were female (76.7%) and White, non-Hispanic (93%), with a mean age of 40.6 years.

Intervention
-
Active device: 810 nm near-infrared light pulsed at 40 Hz
-
Six transcranial LEDs + one intranasal LED
-
Targets DMN hubs and cerebellum
-
-
Sham device: Identical in appearance and sound, but no light output on scalp contact
-
Regimen:
-
20-minute sessions
-
Once daily, 6 days per week
-
8 weeks (56 days) of treatment
-
Additional 4-week follow-up (to Day 84)
-
Participants were trained via video and manual, and adherence was monitored via electronic diaries and device logs.


Primary Outcome: Cognitive Performance
The primary endpoint was change in a composite cognitive score at Day 56, measured by a seven-task Creyos Research (formerly Cambridge Brain Sciences) battery. This composite captured key domains:
-
Short-term and working memory
-
Executive function and reasoning
-
Attention and visual memory
Scores were converted to Z-scores and averaged to produce a global composite index of cognitive performance.
Key Findings
-
In the overall sample, the active PBM group showed a greater mean improvement in composite cognitive score than sham by Day 56:
-
Active: +0.050
-
Sham: +0.007
-
Between-group difference: 0.043 (95% CI: –0.007 to 0.092; p = 0.088)
-
Effect size overall: small (Cohen’s d ≈ 0.28)
-
Although this did not reach conventional statistical significance, it indicates a positive trend favoring active PBM.


Age-Stratified Results
A pre-specified subgroup analysis revealed a clearer signal:
-
Participants <45 years (n = 31):
-
Active: +0.082
-
Sham: +0.023
-
Difference: 0.059 (SE 0.025; 95% CI: 0.007–0.111; p = 0.028)
-
Effect size: medium (Cohen’s d ≈ 0.44)
-
-
Participants ≥45 years:
-
Active: +0.026
-
Sham: +0.016
-
Difference: 0.010 (negligible effect, not significant)
-
In short, younger adults showed the strongest and statistically significant cognitive gains with PBM, whereas older adults showed a smaller, non-significant advantage.


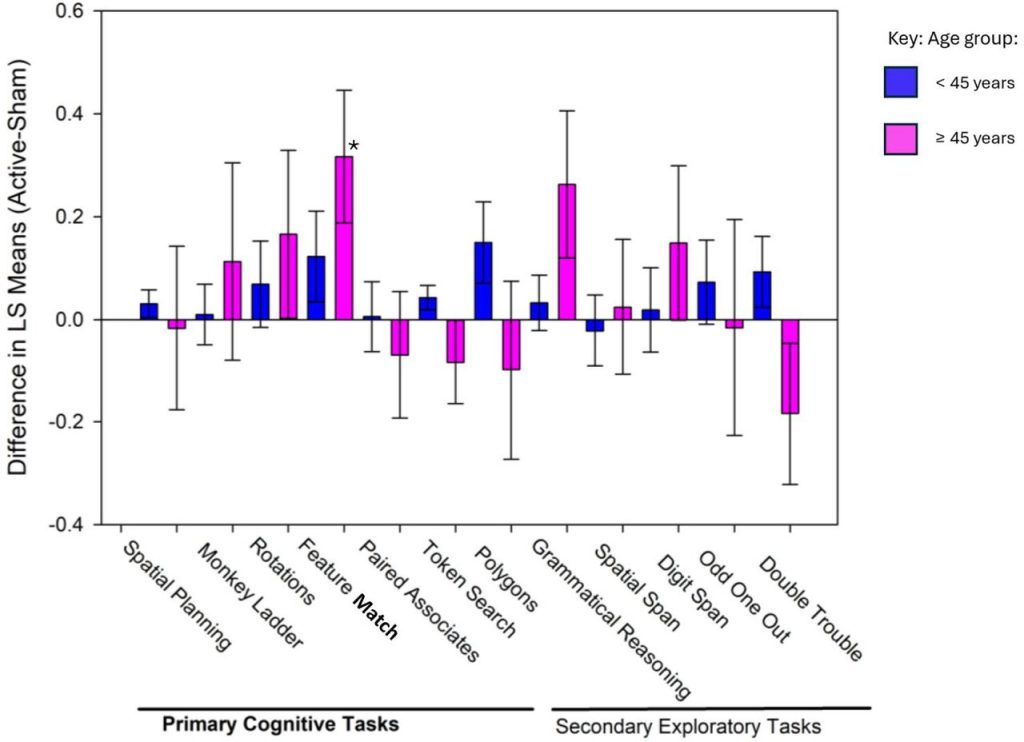
Component Cognitive Tasks: Attention Stands Out
When individual tasks were examined:
-
Six out of seven core tasks numerically favored active PBM at Day 56.
-
The most robust and consistent effect was seen in Feature Match, a task of visual attention and pattern recognition.
Across timepoints:
-
In the full sample, Feature Match accuracy was significantly better in the active group at Day 28 and Day 84.
-
In participants ≥45 years, active PBM outperformed sham on Feature Match at Day 28 and Day 56.
-
In the <45 group, performance consistently favored the active group and reached statistical significance at Day 84.
These findings are consistent with the hypothesis that PBM, particularly at 40 Hz, can modulate attentional networks and gamma oscillations.
Secondary Outcomes: Mixed Signals Beyond Cognition
Quality of Life (EQ-5D-5L)
-
Both groups showed modest changes in overall quality of life with no clear advantage for PBM on the EQ-5D index by Day 56.
-
The sham group, particularly younger sham participants, actually showed greater improvements in mobility (p = 0.007).
Fatigue (Fatigue Assessment Scale)
-
Fatigue outcomes tended to favor sham rather than active PBM.
-
In several fatigue items (e.g., “I am bothered by fatigue,” “I get tired very quickly”), sham participants reported greater improvement by Day 56.
One plausible interpretation offered by the authors is that as brain fog lifts and cognitive function improves, participants may become more aware of their residual physical fatigue, especially given PCC’s limited energy reserves. Cognitive engagement might also reallocate energy away from physical activity in the short term.
Perceived Cognitive Symptoms (PDQ-20) and Symptom Burden (MSBQ)
-
Perceived cognitive difficulties improved in both groups, with few significant between-group differences.
-
A notable exception: younger PBM participants reported less difficulty remembering names at Day 56.
-
Symptom burden decreased slightly in both groups, with some domains (especially in older participants) favoring sham during the treatment period, then partially converging by Day 84 as PBM participants continued to improve post-treatment.

Safety, Tolerability, and Feasibility
The intervention was safe and well tolerated:
-
No serious adverse events occurred.
-
The most common AEs were mild headache (16.3%) and skin irritation (14.0%), all resolving without intervention.
-
One case of nasal discomfort in the active group resolved with saline gel.
-
Adherence was high: median 45 of 48 sessions completed, and 90% of participants achieved >80% compliance.
The fully remote design – including device shipment, online cognitive testing, and electronic reporting – supports the feasibility of home-based itPBM for PCC.
How Should We Interpret These Findings?
This pilot trial supports several key conclusions:
-
Signal of Cognitive Benefit, Especially in Younger Adults
-
Active itPBM with the Vielight Neuro RX Gamma produced greater improvements in composite cognitive performance than sham, with statistically significant gains in participants under 45 years.
-
The most consistent gains appeared in attention, aligning with PBM’s proposed effects on gamma oscillations and cortical network function.
-
-
Age-Dependent Response
-
The stronger response in younger participants may reflect higher baseline neuroplasticity.
-
Older adults may require longer duration, higher doses, or continuous treatment to achieve similar effects.
-
-
Complex Interaction with Fatigue and Quality of Life
-
While cognition improved, some fatigue and mobility measures favored sham, suggesting that early cognitive recovery may unmask or reframe patients’ experience of physical limitations.
-
The pattern in older adults—improved cognition during treatment with some decline after stopping—echoes observations from prior PBM studies in neurodegenerative populations, hinting that continuous or maintenance dosing may be important in certain subgroups.
-
-
Strong Safety and Practicality Profile
-
The absence of serious AEs, high adherence, and fully remote feasibility are important strengths, especially for a chronic condition like PCC where clinic access can be limited.
-
Take-Home Message
This randomized, double-blind, sham-controlled pilot trial suggests that home-based intranasal + transcranial photobiomodulation with the Vielight Neuro RX Gamma is safe, feasible, and associated with meaningful cognitive improvements—particularly in younger adults with long COVID brain fog.
While secondary outcomes were mixed and the overall effect size was modest, the attentional gains and age-stratified results provide a strong rationale for larger, adequately powered trials to fully evaluate PBM as a non-invasive therapeutic option for PCC-related cognitive dysfunction.