Default Mode Network Photobiomodulation


To discuss photobiomodulation and the brain’s default mode network we reached out to Prof. Margaret Naeser, at the VA Boston Healthcare System. She is a Research Professor of Neurology, Boston University School of Medicine. She kindly provided us with some in-depth, detailed information. We asked her to answer a few questions related to her research in photobiomodulation. We actually asked her the same three questions that we asked Prof. Michael Hamblin and Prof. Jay Sanguinetti. Prof. Naeser had a lot to share with us. We decided to split her answers into two parts. This is part one.
Q: What is photobiomodulation in general?
Photobiomodulation (PBM) therapy is a safe, painless, noninvasive, nonthermal modality. It involves the use of primarily red, and/or near-infrared (NIR) wavelengths of light, approximately 600–1100 nm, to stimulate, heal, and repair damaged or dying cells and tissues. Multiple benefits are associated with application of red/NIR PBM to poorly functioning (compromised) cells that are low on oxygen (hypoxic). This includes increased production of adenosine tri-phosphate (ATP) by the mitochondria. Adequate levels of ATP are important for normal cellular energy and respiration.
There is also increased local blood flow after release of nitric oxide from cytochrome C oxidase in the hypoxic cells. Perhaps to put it more simply, PBM may promote a form of “self-healing” for damaged cells. No negative side effects or serious adverse events have been reported, since initial studies for wound healing began in the 1960’s by Endre Mester, MD in Budapest, Hungary.
Q: What is transcranial photobiomodulation specifically?
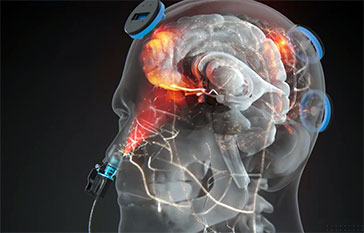
Transcranial PBM (tPBM) is the application of, primarily, near-infrared (NIR) wavelengths of light (for example, 810nm, 830nm, etc.) to the scalp, using light-emitting diodes (LEDs) or low-level laser therapy (LLLT). The goal of tPBM is to deliver enough NIR photons to the scalp, so that NIR photons will reach the surface brain cortex areas below the scalp placement areas. Perhaps only 2 to 3% of the photons will reach the surface brain cortex (Wan, Parrish, Anderson, Madden, 1981). Studies show the depth of penetration of some NIR (808 nm) photons into the brain, reach up to 4-5 cm (Tedford et al., 2015). The NIR photons are hypothesized to improve cellular function in damaged brain cells. These damaged brain cells are likely low on oxygen and functioning poorly.
Traumatic brain injury and transcranial photobiomodulation
When a traumatic brain injury (TBI) occurs, there is damage to nerve cells in brain cortex. There is also damage to the deeper white matter (axons) that connect specific brain cortex areas to each other. These connections are important for normal thinking and memory. When brain cortex is damaged, along with damage to the deeper white matter brain connections, cognitive tasks, such as problem solving and multi-tasking (executive function), cannot be performed with efficiency.
The brain anatomy and physiology relevant to Traumatic Brain Injury (TBI)
The frontal lobes, located behind the forehead and deep to the front sides of the head, are often damaged in TBI. An area of each frontal lobe, located closer to the middle of the brain, is the mesial prefrontal cortex (mPFC) area. This area of the brain has a high demand for glucose and energy in order to function properly (Raichle, 2015; Mormino et al., 2011).
Default Mode Network (DMN) and Traumatic Brain Injury (TBI)
The mPFC is part of an important neural network, the Default Mode Network (DMN). The DMN has two cortical “node” areas (collection of nerve cells) located near the midline (middle) of the brain. One is the mPFC (in the frontal lobes) and the second is the precuneus (in the parietal lobes, behind the frontal lobes). These cortical nodes are “active” when a person is daydreaming or sleeping. However, in order for executive function to take place, these two nodes (mPFC and precuneus) must down-regulate (de-activate) simultaneously. This must occur, in order to permit up-regulation (activation) of other parts of the frontal lobes, such as the dorsolateral prefrontal cortex (dlPFC) on the sides of the frontal lobes, in order to perform executive functions.
However, after TBI, the “nodes” of the DMN are often dysfunctional and cannot “turn off” or down-regulate, simultaneously. Thus, they prevent up-regulation of the dlPFC parts of the frontal lobes which are necessary for executive function and normal brain function. Poor cellular function in the mPFC following TBI can have devastating effects on cognition, including poor executive function. One goal in using tPBMto treat chronic TBI cases is to deliver NIR photons to poorly functioning cells in the cortical “nodes” of the DMN – especially the mPFC and precuneus. The mPFC location, at the center front hairline area on the forehead, makes it an especially vulnerable place for head impact and brain damage.
Additional brain dysfunction related to TBI
In TBI there is often twisting and shearing of the white matter axons, due to the angular force of the head trauma. This type of brain damage is also present after exposure to the blast from an improvised explosive device (IED) that exploded within 100 yards of someone. Ultimately (based on animal studies), this blast wave produces poor mitochondrial function in the nerve cells. Furthermore, there is low production of ATP, as well as lower cerebral blood flow to that part of the brain.
Can a brain with TBI benefit from transcranial photobiomodulation?
After tPBM application of NIR photons to the damaged brain areas, the ATP levels are expected to increase, as well as local blood flow to the area due to release of nitric oxide. Several research labs have shown increased, local cerebral blood flow after tPBM (Schiffer et al., 2009; Nawashiro et al., 2012; Naeser, Ho, Martin et al., 2012; Ho, Martin, Yee et al., 2016; Hipskind et al., 2019; Chao, 2019).
Thus, following tPBM treatments, there is increased cerebral blood flow near the areas treated. Furthermore, the damaged cells begin to function more normally, with increased production of ATP. Our research has observed that in chronic TBI cases after a series of 18 red/NIR tPBM treatments (3 times per week, six weeks), post-testing scores showed significant improvements in executive function and verbal memory, as well as reduced symptoms of PTSD (Naeser, Zafonte et al., 2014; Naeser, Martin, Ho et al., 2016; Naeser, Saltmarche et al., 2011). These improvements were present at 1 week after the final, 18th, tPBM treatment. Also, there was additional improvement 1 month and 2 months later, without any intervening tPBM treatments, in these chronic TBI cases.

How the use of transcranial photobiomodulation is different for TBI and stroke?
In TBI, there is damage to both sides of the brain, due to the twisting and shearing of the axons during the TBI event. In stroke patients, however, there is usually brain damage to only one side of the brain, where the stroke occurred. Thus, in TBI cases we apply the tPBM to both sides of the head. However, in stroke cases, we apply tPBM to only the side of the head where the stroke occurred – i.e., where the compromised/hypoxic cells are located. (Naeser, Ho, Martin, et al., 2012; Ho, Martin, Yee et al., 2016; Naeser, Ho, Martin et al., PMLS in press.)